-40%
Pulse Oximeter Finger Heart Rate SpO2 Monitor PR PI RR Respiratory Rate FDA&CE
$ 5.27
- Description
- Size Guide
Description
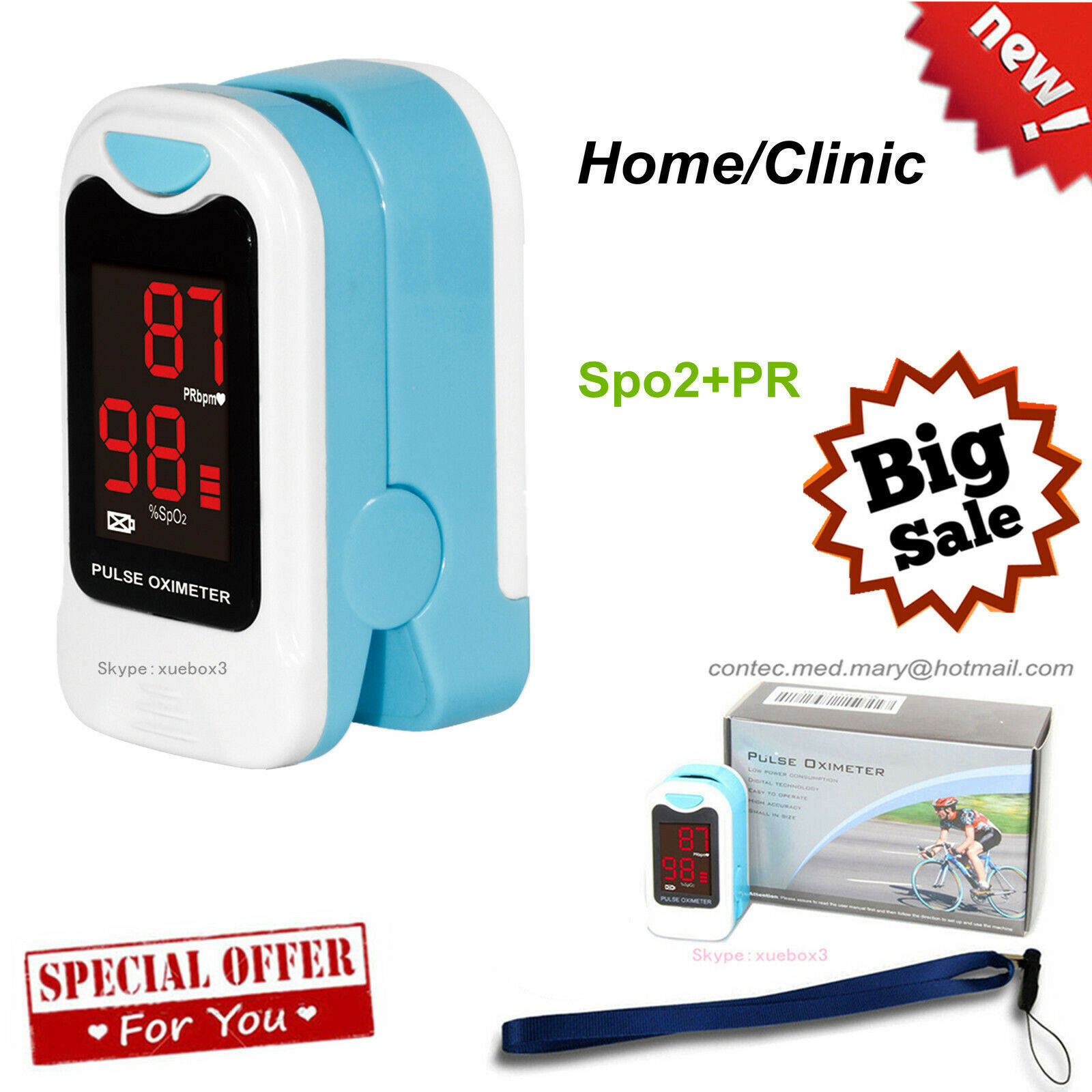
CE &FDAThe pulse oximeter,based on all digital technology, is intended for noninvasive spot-check measurement of functional oxygen stautation of arterial hemoglobin(SpO2).Advanced DSP algorithm can reduce the influence of motion artifact and improve measurement accuracy of low perfusion.
Features for
Pulse Oximeter
:
- Color OLED display, simultaneous display for testing value and plethysmogram.
- The display interface of OLED can rotate four directions whith six different display modes after pressing the power button for less than 0.5s.
- Real-time spot-checks
- Pulse waveform & bargraph display
- Advanced setting for alarm
- Functions of audio alarm and pulse sound
- Automatic power off
- Low power consumption down to 0.3%
- 50 hours continuous to work
- Low perfusion
≤
0.4%
- Low voltage indicator
- Widely used in hospital, home healthcare, oxygen bar, community medical centre, alpine area, sports healthcare etc.
Specification for
Pulse Oximeter
:
Specification:
Display
OLED two color display
SpO2
Measurement range: 70
~
99%
Resolution: ±1%
Accuracy: ±2% (70%
~
99%), unspecified (<70%)
Pulse rate
Measurement range: 30
~
240 bpm
Resolution: ±1%
Accuracy: ±2bpm or ±2% (select larger)
Low Perfusion ≤0.4%
Power
1.5V (AAA size)
alkaline battery x 2
Supply voltage: 2.6~3.6V
Working current
≤30mA
Automatic power-off
Automatically power off when no signal in the oximeter for more than 8 seconds
Dimension & Weight
58 (L) × 31(W) × 29(H) mm
60g
Package Content:
-
1 x RPO-8B5 Fingertip Oximeter
-
1 x Lanyard
-
1 x English user's manual
Note:
We will not include batteries, according to
China law, batteries are not allowed for international air transportation.
We only accept payment via PayPal.
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(CICI-China-86-18328503374)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892